West Nile Virus: Virus, Disease, Public Health and Product Efficacy Testing
Thousands of cases of human West Nile Virus (WNV) have been reported in 2018. The total number exceeds the total reported cases in the last 7 years combined in the European Union/European Economic Area (EU/EEA)6. This article gives an overview of the virus, disease, and testing methodologies for disinfectants and antiseptics for efficacy against WNV.
Microbac Laboratories, Inc. has successfully developed an assay for WNV and now offers regulatory-compliant virucidal efficacy testing for disinfectants and antiseptics that seek an official claim against WNV.
Recent West Nile Virus Outbreak and Symptoms
The West Nile Virus is an arbovirus, a virus transmitted by arthropods and similar to many flaviviruses. It is maintained in nature through a mosquito-bird-mosquito transmission cycle, mosquitoes (specifically Cx. Pipiens) infect humans during blood meals6.
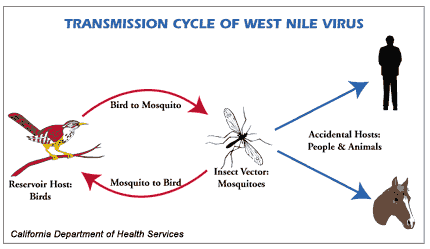 For the 2018 transmission season, the reported cases of West Nile Virus in Europe dwarfed that of previous seasons. 503 human cases were reported in the EU/EEA, along with 580 human cases reported by EU neighboring countries. The total reported deaths due to West Nile Virus was 180. The total number of reported cases for 2018 increased 7-fold from 2017. In 2018, the number of reported equine infections rose 30% from 20176. These outbreaks highlight the danger of mosquito-borne diseases, which are able to implant themselves outside the endogenous habitat of the organism.
For the 2018 transmission season, the reported cases of West Nile Virus in Europe dwarfed that of previous seasons. 503 human cases were reported in the EU/EEA, along with 580 human cases reported by EU neighboring countries. The total reported deaths due to West Nile Virus was 180. The total number of reported cases for 2018 increased 7-fold from 2017. In 2018, the number of reported equine infections rose 30% from 20176. These outbreaks highlight the danger of mosquito-borne diseases, which are able to implant themselves outside the endogenous habitat of the organism.
WNV infections have no symptoms in 80% of confirmed cases. The remaining 20% of those infected will develop West Nile fever; the symptoms of which include fever, headache, tiredness, body aches, nausea, vomiting, skin rash, and swollen lymph nodes4. Around 1 in 150 persons develop a more serious infection, leading to West Nile encephalitis/meningitis or West Nile poliomyelitis. The symptoms of these neuroinvasive diseases include headache, high fever, neck stiffness, stupor, disorientation, coma, tremors, convulsions, muscle weakness, and paralysis4.
West Nile Virus Biology
West Nile Virus (WNV) is a Flavivirus, along with similar viruses such as Zika Virus, Dengue Virus, and Yellow Fever Virus.  It was first isolated in Uganda in 1937 and comprises two distinct lineages. The virus is known to infect humans, monkeys, horses, birds, and even cetaceans5. It has an ssRNA genome and an icosahedral capsid. The virus particle is enveloped and is about 50 nm in diameter7. The virus has been shown to be susceptible to photochemical and heat treatment, hydrogen peroxide, and formalin1-3. Outbreaks first occur in animals and are transmitted to humans by bites from an infected mosquito.
It was first isolated in Uganda in 1937 and comprises two distinct lineages. The virus is known to infect humans, monkeys, horses, birds, and even cetaceans5. It has an ssRNA genome and an icosahedral capsid. The virus particle is enveloped and is about 50 nm in diameter7. The virus has been shown to be susceptible to photochemical and heat treatment, hydrogen peroxide, and formalin1-3. Outbreaks first occur in animals and are transmitted to humans by bites from an infected mosquito.
Treatment and Prevention of West Nile Virus
Although equine vaccines have been developed, there is no WNV vaccine available for humans. As such, the treatment is supportive (i.e. symptomatic treatment) in severe cases of the neuroinvasive forms of WNV, and may require hospitalization, intravenous fluids, respiratory support, and prevention of secondary infections6.
The most common cause of infection of WNV in humans is from infected mosquitos, which contract the virus from avian species. However, incidences of transmission from laboratory exposure, blood transfusion, organ donation, and transplacental routes have been reported5. As such, handling of the virus in a laboratory or health care setting requires the use of standard infection control precautions. Disinfecting surfaces in contact with WNV is useful in curbing occupational contraction of the virus.
In humans, prevention revolves around vector (mosquito) control. This includes protection against mosquito bites by use of mosquito nets, insect repellent, minimizing exposed skin, and destruction of mosquito breeding sites. As outbreaks in animals precede human outbreaks, an efficient animal and mosquito surveillance program is crucial in preventing the spread of WNV.
Efficacy Testing for Disinfectants / Antiseptics Against West Nile Virus
 Microbac Laboratories, Inc. is a leading contract testing laboratory for antimicrobial/antiviral testing for disinfectants, antiseptics and medical devices. With more than 28 years of experience in the industry, we are excited to announce that we now offer regulatory-compliant testing against WNV.
Microbac Laboratories, Inc. is a leading contract testing laboratory for antimicrobial/antiviral testing for disinfectants, antiseptics and medical devices. With more than 28 years of experience in the industry, we are excited to announce that we now offer regulatory-compliant testing against WNV.
Disinfectants should be tested following the ASTM E1053-11 method for U.S. EPA or Canada submission; or the EN14476 method for European Union submission. Antiseptics should be tested following ASTM E1052-11 or EN14476. All these tests are readily available at Microbac, under GLP, non-GLP, or cGMP depending on the goal of the project. Our laboratories are ISO 17025 accredited and offer excellent regulatory compliance, quality, cost competitiveness and fast turn-around to our clients.
 For the 2018 transmission season, the reported cases of West Nile Virus in Europe dwarfed that of previous seasons. 503 human cases were reported in the EU/EEA, along with 580 human cases reported by EU neighboring countries. The total reported deaths due to West Nile Virus was 180. The total number of reported cases for 2018 increased 7-fold from 2017. In 2018, the number of reported equine infections rose 30% from 20176. These outbreaks highlight the danger of mosquito-borne diseases, which are able to implant themselves outside the endogenous habitat of the organism.
For the 2018 transmission season, the reported cases of West Nile Virus in Europe dwarfed that of previous seasons. 503 human cases were reported in the EU/EEA, along with 580 human cases reported by EU neighboring countries. The total reported deaths due to West Nile Virus was 180. The total number of reported cases for 2018 increased 7-fold from 2017. In 2018, the number of reported equine infections rose 30% from 20176. These outbreaks highlight the danger of mosquito-borne diseases, which are able to implant themselves outside the endogenous habitat of the organism. It was first isolated in Uganda in 1937 and comprises two distinct lineages. The virus is known to infect humans, monkeys, horses, birds, and even cetaceans5. It has an ssRNA genome and an icosahedral capsid. The virus particle is enveloped and is about 50 nm in diameter7. The virus has been shown to be susceptible to photochemical and heat treatment, hydrogen peroxide, and formalin1-3. Outbreaks first occur in animals and are transmitted to humans by bites from an infected mosquito.
It was first isolated in Uganda in 1937 and comprises two distinct lineages. The virus is known to infect humans, monkeys, horses, birds, and even cetaceans5. It has an ssRNA genome and an icosahedral capsid. The virus particle is enveloped and is about 50 nm in diameter7. The virus has been shown to be susceptible to photochemical and heat treatment, hydrogen peroxide, and formalin1-3. Outbreaks first occur in animals and are transmitted to humans by bites from an infected mosquito. Microbac Laboratories, Inc. is a leading contract testing laboratory for
Microbac Laboratories, Inc. is a leading contract testing laboratory for